 |
 |
Last Update: September 09th, 2025
SUBMISSION AND PEER REVIEW PROCEDURE
TYPES OF PUBLICATION ACCEPTED AND FORMATTING GUIDELINES
****SUBMISSION OF FILES WILL BE DONE EXCLUSIVELY BE GOOGLEFORMS AFTER REGISTRATION ON THE PLATFORM (BOTH ARE NECESSARY)***
Submission Procedure:
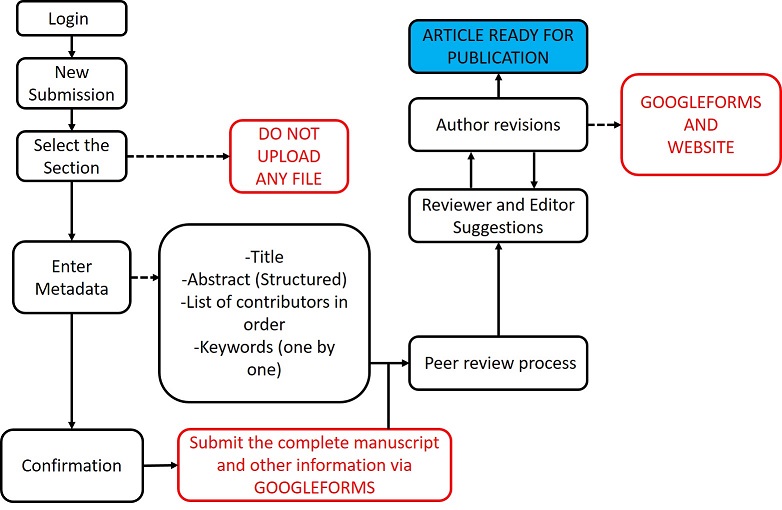
Please note: After acceptance of the article, a contribution of US$7.00 is required to generate a permanent Digital Object Identifier (DOI)
Revision Procedure
APN uses a double blind blind peer review system where reviewers do not know the names of the authors, and the authors do not know who reviewed their manuscript.
Please check Peer review process for further information
After submitting the 'Revision Letter' on the platform, MANUSCRIPT REQUIRED REVISIONS MUST BE SUBMITTED THROUGH THE GOOGLE FORMS REVISION FORM!
Author contributions
For transparency, APN demand authors to submit an author statement outlining their individual contributions to the paper using the relevant CRediT roles: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Roles/Writing - original draft; Writing - review & editing. Authorship statements should be formatted with the names of authors first and CRediT role(s) following. The author's statement of contribution is submitted during the GOOGLEFORMS filling out.
APN have a restriction in the number of contributions for each type of article, please refer to the table with the information below.
Author names and affiliations
Please clearly indicate the given name(s) and family name(s) of each author and check that all names are accurately spelled. You can add your name between parentheses in your own script behind the English transliteration. Affiliations must be in the native language in the following order (if applicable): section, department, institution, City, State and Country. Please submit in English the name of the institution in parentheses.
Statement of Ethics
Archives of Pediatric Neurosurgery adheres to the ethical standards described by the Committee on Publication Ethics (COPE) and the International Committee of Medical Journal Editors (ICMJE). Archives of Pediatric Neurosurgery is also listed in the ICMJE list of journals following their recommendations. Editors, Authors, and Reviewers are expected to adhere to these standards.
The editors of APN are committed to using COPE’s flowcharts (https://publicationethics.org/guidance/Flowcharts) in case of suspected ethical violations related to the following cases of suspected misconduct: duplicate publication; plagiarism; data fabrication; undisclosed conflict of interest; as well as any other suspicious ethical problems of submitted papers.
The editors are committed to ensuring fair play and confidentiality principles while handling papers submitted to APN. They are also responsible for advising reviewers on using those same principles for evaluating contributions assigned to them. Reviewers should promptly send their reviews using relevant and well-structured arguments to help the authors improve their paper.
Authors should ensure their paper follows ethical principles, especially regarding:
Human Subjects
Manuscripts that involve research conducted on human subjects must follow the principles outlined in the Declaration of Helsinki and include a statement in the Methods section stating that the experimental protocol and informed consent were approved by the Institutional Review Board (IRB), and that all subjects gave informed consent. If IRB approval or patient consent was not sought or obtained, authors should include an explanation in the Methods section. Authors should indicate the mechanism used for reviewing the ethics of the research conducted in their cover letter.
Animal Subjects
Manuscripts that report animal experiments must include a statement in the Methods section stating that the study was approved by the Institutional Review Board and that the animal care complied with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. Washington: National Academy Press, 1996). Authors should indicate the mechanism used for reviewing the ethics of the research conducted in their cover letter.
Note: Though not required at the time of submission, authors should be prepared to provide evidence of IRB/Ethics Committee adherence if requested by the Editor.
Patient Consent
It is the policy of the Journal that no identifiable protected health information (PHI) of any person may be included in any manuscript submitted to or published by Archives of Pediatric Neurosurgery publications unless the information is essential for the scientific integrity or purpose of the manuscript or published work and the patient has given written informed consent. This policy includes, but is not limited to, any identifiable protected health information subject to applicable laws and regulations concerning the privacy and/or security of personal information.
Authors should pay close attention to images that contain identifiable individual patient characteristics or data such as eyes, date of birth, case number, initials, birthmarks, scars, tattoos, piercings, etc. Care should be taken where an individual’s head or face appears, or where reference is made to an individual’s name or other personal details. Particular care should be taken where children are concerned.
Author(s) shall take all steps necessary to ensure that (1) there is no PHI contained in any text, data, or images in the manuscript unless that PHI is necessary to the scientific integrity of the publication and the patient has provided consent to the publication of their PHI; and (2) all other pre-existing PHI, if any, has been fully anonymized and de-identified (see below for how to properly anonymize and de-identify data).
As a condition of submission to the Archives of Pediatric Neurosurgery Publications, all authors warrant that he or she has obtained, prior to submission, written releases from patients whose names or likenesses are submitted as part of the Work. Should the Journal or Publisher request copies of such written releases, authors shall provide them in a timely manner. In addition to the foregoing requirements, each author must ensure that each authorizing individual, or the individual's legal guardian or other person with legal authority to act on the individual's behalf, who may be identified in any video, recording, photograph, image, illustration or case report (or in any other identifiable form) relating to a proposed manuscript is made aware in advance of the fact that such photographs are being taken or such video, recording, photograph, image, illustration or report is being made, and of all the purposes for which they might be used, including disclosure to Archives of Pediatric Neurosurgery Publications and use by Archives of Pediatric Neurosurgery Publications and any affiliated publication. Such individual, legal guardian, or person with legal authority must give his/her explicit written authorization in writing. If authorization has been obtained, care must be taken to ensure that the portrayals and captioning of any individual are respectful and could not be seen as denigrating that individual. In the case of images of a child, it is important to ensure that images show only children in a suitable dress in order to reduce the risk of images being used inappropriately. If such authorization is made subject to any conditions (for example, adopting measures to prevent personal identification of the person concerned), APN Publications must be made aware in writing of all such conditions. If an author does not obtain the written authorization of the individual or the individual’s legal guardian, the video, etc. cannot be used in the Work.
Patient Consent form - English
Manuscript format
|
Article Type |
Abstract Limit |
Keywords Limit |
Title Limit |
Tables/Figures Limit |
References Limit |
Peer reviewed |
Maximum number of authors |
|
Review Article (up to 3,000 words) |
Up to 250 words |
Up to 5 keywords |
Up to 25 words |
Approximately 7 tables/figures |
Up to 90 references |
yes |
6 |
|
Original Article (up to 3,000 words) |
Up to 250 words |
Up to 5 keywords |
Up to 25 words |
Approximately 5 tables/figures |
Up to 50 references |
yes |
6 |
|
Clinical Case Reports (up to 2,500 words) |
Up to 250 words |
Up to 5 keywords |
Up to 25 words |
Up to 5 tables/figures |
Up to 15 references |
yes |
6 |
|
How I do it (up to 1200 words) |
up to 100 wods |
Up to 5 keywords |
Up to 25 words |
Up to 10 tables/figures |
Up to 15 references |
yes |
2 |
|
Clinical Images (up to 200 words) |
n/a |
Up to 5 keywords |
Up to 25 words |
Legend: up to 200 words |
Up to 5 references |
yes |
4 |
|
Clinical Videos (up to 200 words) Maximum length 5 minutes |
n/a |
Up to 5 keywords |
Up to 25 words |
Legend: up to 100 words |
Up to 5 references |
yes |
4 |
|
Editorial |
n/a |
n/a |
Up to 25 words |
n/a |
n/a |
no |
3 |
|
Letter to the Editor |
n/a |
Up to 5 Keywords |
Up to 25 words |
n/a |
n/a |
no |
2 |
|
Technical Notes (Up to 1,000 words) |
Up to 250 words |
Up to 5 keywords |
Up to 25 words |
Approximately 5 tables/figures |
Up to 25 references |
yes |
4 |
|
Historical Vignettes, Obituaries, Etc. (up to 1,200 words) |
n/a |
Up to 5 Keywords |
Up to 25 words |
Approximately 5 tables/figures |
Up to 25 references |
no |
4 |
|
Commentary (Up to 850 words) |
n/a | Up to 5 Keywords | Up to 25 words | Approximately 2 tables/figures | Up to 15 references | no | 2 |
- The number of authors may exceed the limit if there is a valid formal justification provided to the editor-in-chief
****SUBMISSION OF FILES WILL BE DONE EXCLUSIVELY BE GOOGLEFORMS AFTER REGISTRATION ON THE PLATFORM (BOTH ARE NECESSARY)***
Authors are encouraged to use the relevant research reporting guidelines for their study type provided by the EQUATOR Network. This will ensure that you provide enough information for editors, peer reviewers and readers to understand how the research was performed and to judge whether the findings are likely to be reliable.
The key reporting guidelines are:
• Randomised controlled trials (RCTs): CONSORT guidelines, flowchart and structured abstract checklist
• Systematic reviews and meta-analyses: PRISMA guidelines, flowchart and structured abstract checklist
• Observational studies in epidemiology: STROBE guidelines (also refer to RECORD for observational studies using routinely collected health data) and MOOSE guidelines
• Diagnostic accuracy studies: STARD guidelines
• Quality improvement studies: SQUIRE guidelines
• Multivariate prediction models: TRIPOD guidelines
• Economic evaluation studies: CHEERS guidelines
• Animal pre-clinical studies: ARRIVE guidelines
Article Types
The following paragraphs show what types of articles are accepted for publication, and their requirements.
Commentaries are invited at the discretion of the Editor-in-Chief and can be a brief communication on a subject pertinent to the field. The goal of a commentary in this case is to enrich the reader's understanding of an issue by highlighting a particular aspect of the given article or to offer an alternative perspective on the contents reported.
Commentaries are short, narrowly focused articles of contemporary interest and usually take one of the following forms:
General Guidelines
Article structure
Manuscript
Acknowledgments
The source of any financial support received and recognition of personal assistance for the work being published should be indicated at the end of the article, just before the Reference section, under the heading
“ Acknowledgments”.
Conflict of Interest
It is required that a list of disclosures from every named author is submitted alongside the manuscript. In it, each author should identify any financial or non-financial conflicts relevant to the article. If no conflicts exist, please state so in this section.
Types of conflicts include: Consulting, Royalties, Research Support, Institutional Support, Ownership, Stock/Options, Speakers Bureau, and Fellowship Support. Any commercial entity whose products are described, reviewed, evaluated, or compared in the manuscript, except for those disclosed in the Acknowledgments section, are potential conflicts.
References
References should be the most recent and pertinent literature available. It is essential that they are complete and thoroughly checked. If the reference information is incomplete, good online sites to search for full details are the National Library of Medicine: www.nlm.nih.gov; Books in Print: www.booksinprint.com; PubMed: www.ncbi.nlm.nih.gov/PubMed/; or individual publisher Web sites.
Endnote: https://endnote.com/wp-content/uploads/plugins/styles/Vancouver.ens
References should be styled per the following examples:
de Oliveira RS, Cinalli G, Roujeau T, Sainte-Rose C, Pierre-Kahn A, Zerah M. Neurenteric cysts in children: 16 consecutive cases and review of the literature. Journal of Neurosurgery: Pediatrics. 2005 Dec 1;103(6):512- 23.
Toma H. Takayasu’s arteritis. In: Novick A, Scoble J, Hamilton G, eds. Renal Vascular Disease. Philadelphia: WB Saunders; 1995:47–62
Stryer L. Biochemistry. 2nd ed. San Francisco: WH Freeman; 1981:559–596
Stern I. Hemorrhagic Complications of Anticoagulant Therapy [Ph.D. dissertation]. Evanston, IL: Northwestern University; 1994
Food and Drug Administration. Jin Bu Huan Herbal Tablets. Rockville, MD: National Press Office; April 15, 1994. Talk Paper T94-22
Rosenthal S, Chen R, Hadler S. The safety of acelluler pertussis vaccine vs whole-cell pertussis vaccine [abstract]. Arch Pediatr Adolesc Med [serial online]. 1996;150:457–460. Available at: http://www.ama-assn.org/sci-pubs/journals/archive/ajdc/vol_150/no_5/abstract/htm. Accessed November 10, 1996
Eisenberg J. Market forces and physician workforce reform: why they may not work. Paper presented at: Annual Meeting of the Association of American Medical Colleges; October 28, 1995; Washington, DC
Figure Captions
Tables
General Guidelines
Note: Lower resolutions (less than 300 dpi) and JPEG format (.jpg extension) for grayscale and color artwork are strongly discouraged due to the poor quality they yield in printing, which requires 300 dpi resolution for sharp, clear, detailed images. JPEG format, by definition, is a lower resolution (compressed) format designed for quick upload on computer screens.
Black-and-White Art
Art Labels
Please contact the Editorial Office Archives of Pediatric Neurosurgery with any questions.
Editorial Office Archives of Pediatric Neurosurgery Phone: +55 51 33882424 / + 55 51 999637811 Email: editorialoffice@sbnped.com.br
When publishing in Archives of Pediatric Neurosurgery journal, authors retain the copyright of their article and agree to license their work using a Creative Commons Attribution 4.0 International Public License (CC BY 4.0), thereby accepting the terms and conditions of this license (https://creativecommons.org/licenses/by/4.0/legalcode).
The CC BY 4.0 license terms applies to both readers and the publisher and allows them to: share (copy and redistribute in any medium or format) and adapt (remix, transform, and build upon) the article for any purpose, even commercially, provided that appropriate credit is given to the authors and the journal in which the article was published.
Authors grant Archives of Pediatric Neurosurgery the right to first publish the article and identify itself as the original publisher. Under the terms of the CC BY 4.0 license, authors allow the journal to distribute the article in third party databases, as long as its original authors and citation details are identified.
The names and email addresses entered in this journal site will be used exclusively for the stated purposes of this journal and will not be made available for any other purpose or to any other party.
Advertising and Marketing: APN reserves the right to insert advertisements from sponsors and supporters after evaluation and approval by the Journal Management Editors. Advertisements will not be related in any way to editorial decision-making and will be kept separate from the published content
Editorial Office Archives of Pediatric Neurosurgery
University Hospital, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto/SP, Brazil
Address: 3900 Bandeirantes Avenue, Ribeirao Preto/SP, Brazil. ZIP Code: 14049-900
Phone number: +55 (51) 3388-2424 / + 55 (51) 99963-7811
Email: editorialoffice@sbnped.com.br
ISSN: 2675-3626